April 14, 2020
Case Report: Intensive Lokomat® Training in a Chronic Complete Spinal Cord Injured Patient
Ignacio Montoya, Executive Director,
HINRI Labs, Atlanta, USA
We would like to share our clinical experience with a patient with a paraplegia due to a complete SCI. The patient arrived to our clinic 5 years after his diagnosis.
An intensive training based only on Robotic Assisted Gait Training with the Lokomat® was provided in our facility for three months. The treatment was separated in two phases with increasing challenges.
The measurable improvements we have documented and observed during the first phase of the locomotor training protocol have been promising and encouraging. Our intent is to continue documenting our progress and to use this data along with our partnerships.
These findings have motivated us to share how the Lokomat can be incorporated into a program with a complete SCI patient population.
Pre-Treatment
A 27-year-old patient, masters degree student came to our facility with a diagnosis of a T4 SCI ASIA A.
The patient presented a paraplegia due to complete SCI, 5 years post injury.
On the right upper extremity there was a nerve transfer of T3, due to a brachial plexus injury. The AIS level for the spinal cord injury (see Fig. 1), takes the brachial plexus injury out of consideration. Without this the level of injury would likely be around C3 as the sensation and strength of the right arm would not be intact. Level A (Fig 1) was indicated as complete: no sensory or motor function was preserved.
The patient has a healthy and active lifestyle, stretching and home exercises have been regularly done.
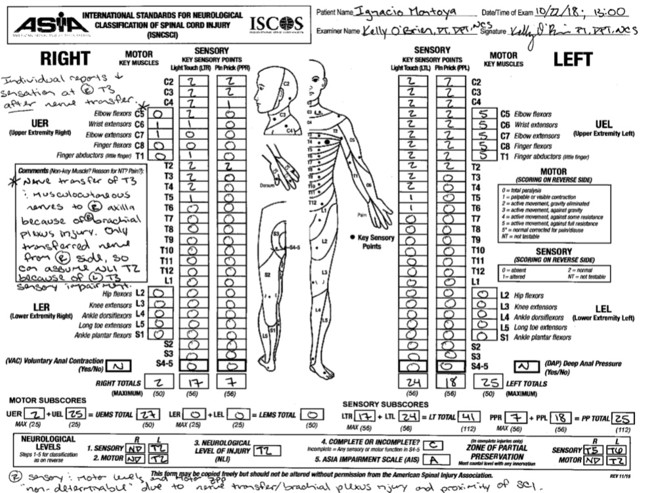
Figure 1: ASIA classification from October 2018. Note that in the comments box the therapist references the right brachial plexus injury. The patient is classified as having a complete SCI as the Sacral 4-5 roots show a “N00N”.
Treatment With the Lokomat® – Phase 1: Decrease of Body Weight Support
The focus of phase 1 of the treatment went from November 2018 until February 2019 and was on decreasing the body weight support (BWS) to 0 kg. The locomotor training schedule consisted of a 3 hour session 3 times per week which lasted for 3 months and made a total of 40 sessions. The only training provided to the patient was intensive gait training with the LokomatNanos. The challenge along the sessions was increased with an increase of the treadmill speed and a decrease of the BWS until 0 kg (Tab. 1).
Table 1: Patient’s therapy session progression.
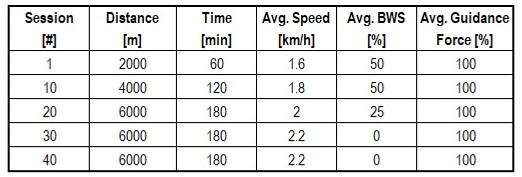
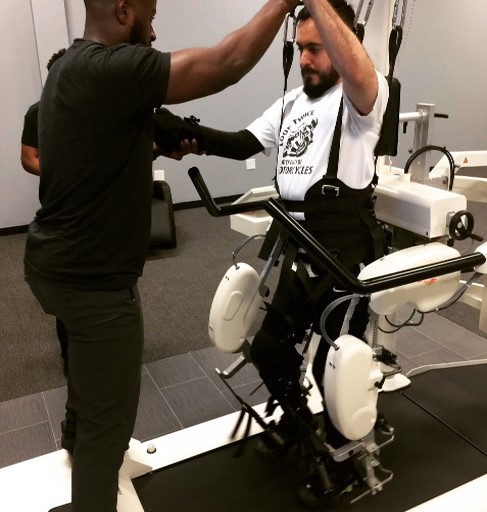
Figure 2: While participating in LokomatNanos training, the patient incorporated upper extremity strengthening in an upright functional position.
Post-Treatment
The Robotic Assisted Gait Training (RAGT) provided by the Lokomat has contributed to a more physiological gait pattern during the Lokomat training through visualization (using a mirror) and intent, e.g. initial contact with heel, and promoted observable emotional and psychological benefits.
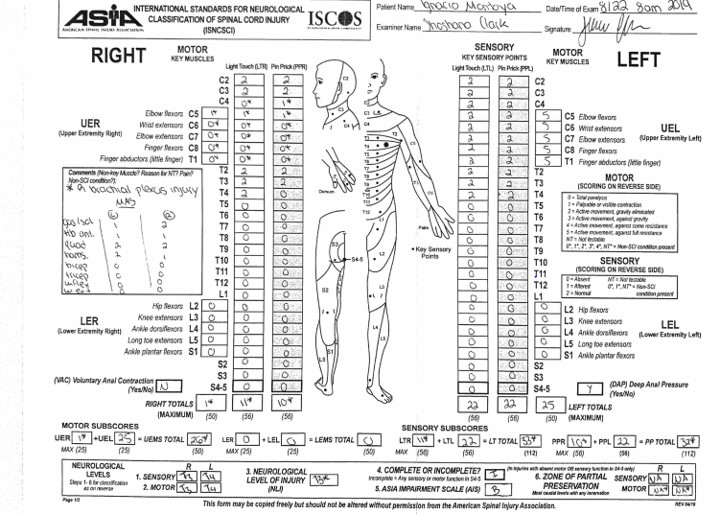
Figure 3: ASIA classification from August 2019. Sacral 4-5 segments now display a “N00Y”, so patient’s ASIA classification is now changed to SCI incomplete AISA B due to improving sensation.
His situational awareness of body positioning in space increased, such as e.g. the ability to tell if the hips are slightly rotated or the harness is positioned a bit off the center. Finally he was always excited and in a high energy levels during every training session.
Video 1: This video shows that the patient receives no BWS and bears 100% of his weight, aside from the assist through the his arms.
Treatment With the Lokomat® – Phase 2: Decrease of the Guidance Force
The 2nd phase of the treatment is currently ongoing. It includes transcutaneous electrical stimulation while walking on the Lokomat and also overground gait training. The main parameter to challenge the patient is a reduction of guidance force. The Locomotor training consists of a 2 hour session twice a day, 5 days a week, for a duraction of 6 months. This makes a total of 250 sessions, geared towards generating voluntary, observable movement below the injury site.
Conclusions
Very intensive therapy resulted in emotional/psychological benefits, among others.
About Us
HINRI Labs (The Healthcare Institute for NeuroRecovery and Innovation) is an independent, private, charitable paralysis recovery laboratory who’s mission is to change the standard of care for the chronic, complete spinal cord injured population. HINRI Labs uses its clinical, military, and university partners to identify, accelerate, and maximize the most promising treatments, technologies, and protocols that have successfully demonstrated the neurological recovery of movement, function, and/or sensation, individually or in combination, after a chronic complete spinal cord injury. Our goal is to compile, combine, and optimize an individualized neuro-recovery formula that will end paralysis and make those safe and efficacious breakthroughs accessible to patients, prove their effectiveness, and change the standard of care across the United States and ultimately the world.
Today we translate translational research into an independent, sustainable, transformed quality of life after paralysis caused by spinal cord injuries, tomorrow we WALK!

This clinical experience report is meant to serve as an example of how the Lokomat is integrated into the rehabilitation program of a chronic complete spinal cord injured patient. It is not necessarily a standard recommendation from Hocoma.